Hi all,
We have been doing a lot of reorganizing of the lab recently in order to make room for two new employees joining us soon: an objects conservator, who will be working on the museum’s collection (not Monitor related) and a chemist, who will be in charge of ALL the analyses we do here. This is very exciting for us on so many levels! For the current Monitor crew, a chemist position means that we will all stop spending almost half of our work time running the ion chromatograph or checking the potential of artifacts in electrolytic reduction… a chemist means sooo much more time for us all to be hands on objects. This is really outstanding and we cannot wait to pass on this work load to someone else!
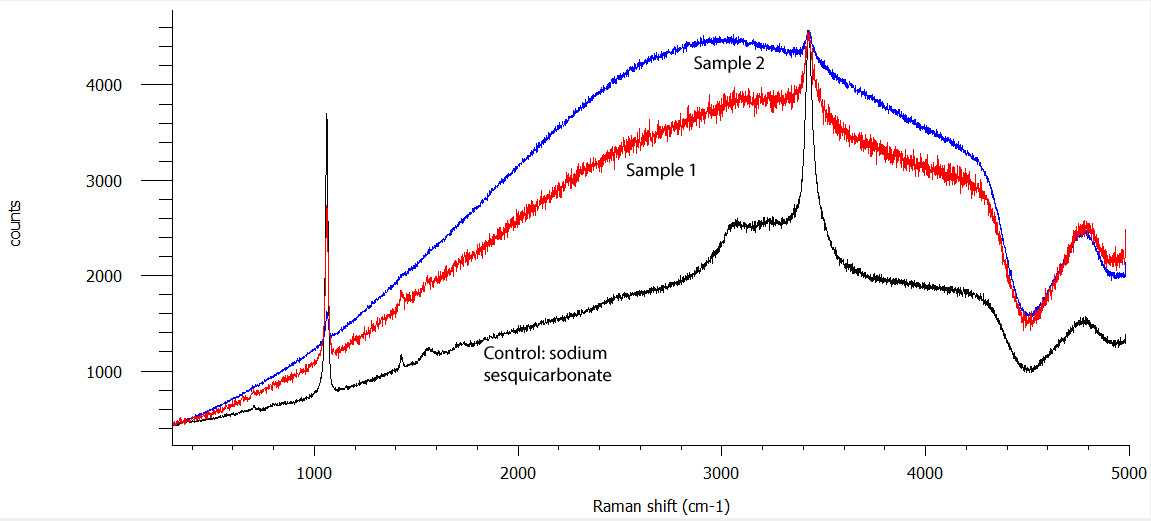
Speaking of chemistry… We have run into an issue with Monitor’s copper alloy artifacts in the past few years. Sometimes after treatment, once the objects are dry, and if the relative humidity in the room exceeds 40%, copper alloy objects are blooming a white powdery compound. Kate presented a poster at a conference a few years back about the issue and was able to identify that sodium was part of the white product. Now, because the electrolyte used for copper alloys here (and in many other labs) is sodium sesquicarbonate (Na3H(CO3)2), we assumed that this was powder was Na3H(CO3)2 that was not sufficiently rinsed and bloomed out of the artifacts once dry. Sodium sesquicarbonate is indeed white.
Hypothesizes are good, but they must be proven or disproven in order to start solving problems… We therefore contacted local universities to find out if an XRD would be available for us to test this powder. XRD or X-ray diffraction is an analytical tool that identifies crystals. It is the instrument that shows how diamond and graphite are different even though they are both composed of carbon… In any event, we located several XRD in the area but ended up not being able to use them because our samples, collected at the surface of the objects were too small!!
Then we asked Olga Trofimova, laboratory and research technician at the Applied Research Center of William and Mary, if we could have access the king of material analyses analytical tool: a Raman spectrometer. This apparatus shoots a laser at a sample. Some of the light passes through the sample and some of the light bounces around between the molecules of the sample and comes out in all directions, which is called scattering. Most of the light that scatters has the same level of energy as the laser, but 1 in every 10 million photons, the particles that make up light, scatters at a different energy than the laser. This is called Raman scattering. Every different type of molecule has its own Raman “fingerprint” making this a great technique for material analysis. Another advantage is that only a very small amount of sample is needed and that it can identify both organic and inorganic material (quite incredible as most instruments analyze either organic or inorganic materials).
Luckily, we were able to take our samples to Williamsburg and have a very nice PhD student run them for us.

What we learned is that it is actually sodium sesquicarbonate blooming at the surface of these objects. “All this for that” you say?! Again, without this confirmation, there was no way for us to even start fixing the problem. Now we can move on and develop a better rinsing process for the copper alloys of the USS Monitor collection!
Stay tuned for more detective/conservation adventures!