We all know that paper isn’t exactly one of the most waterproof materials out there. In fact, water exposure is one of the most common causes of damage to paper objects that I see as a paper conservator. It can cause major issues such as distortion, staining, loss of media, and mold, just to name a few. It’s somewhat counterintuitive, then, that water is a crucial part of many types of conservation treatment. People are often a bit shocked that bathing paper (yes, it’s what it sounds like) is a common practice with positive results! In this post, we will explore a few ways in which water interacts with paper on different levels, how conservators harness and leverage these interactions to treat condition issues, and how, if left unchecked, these interactions can cause major and irreparable damage.
Papermaking – Wet Beginnings

To understand paper’s relationship with water, we have to go back to the beginning – papermaking. True paper is defined as a non-woven mat of fibers formed by draining water from a slurry. The water holds the fibers in a suspension into which a screen called a mold is dipped and drawn upward, allowing the water to drain and the fibers to settle into a layer over the screen. To ensure an even distribution, the mold is shaken back and forth while the water drains, causing many of the fibers to orient in the direction of the movement before coming out of the water. This causes a characteristic called “grain” in a finished piece of paper.
The grain of a paper mainly affects mechanical properties, such as how easily it will fold or tear in a certain direction, but it also affects how subsequent water exposure will affect the paper for the rest of its life! In the presence of moisture (even a relatively small amount) a sheet of paper will expand in all directions from the uptake of water into and between individual fibers, BUT it will expand to a much greater degree across the grain.
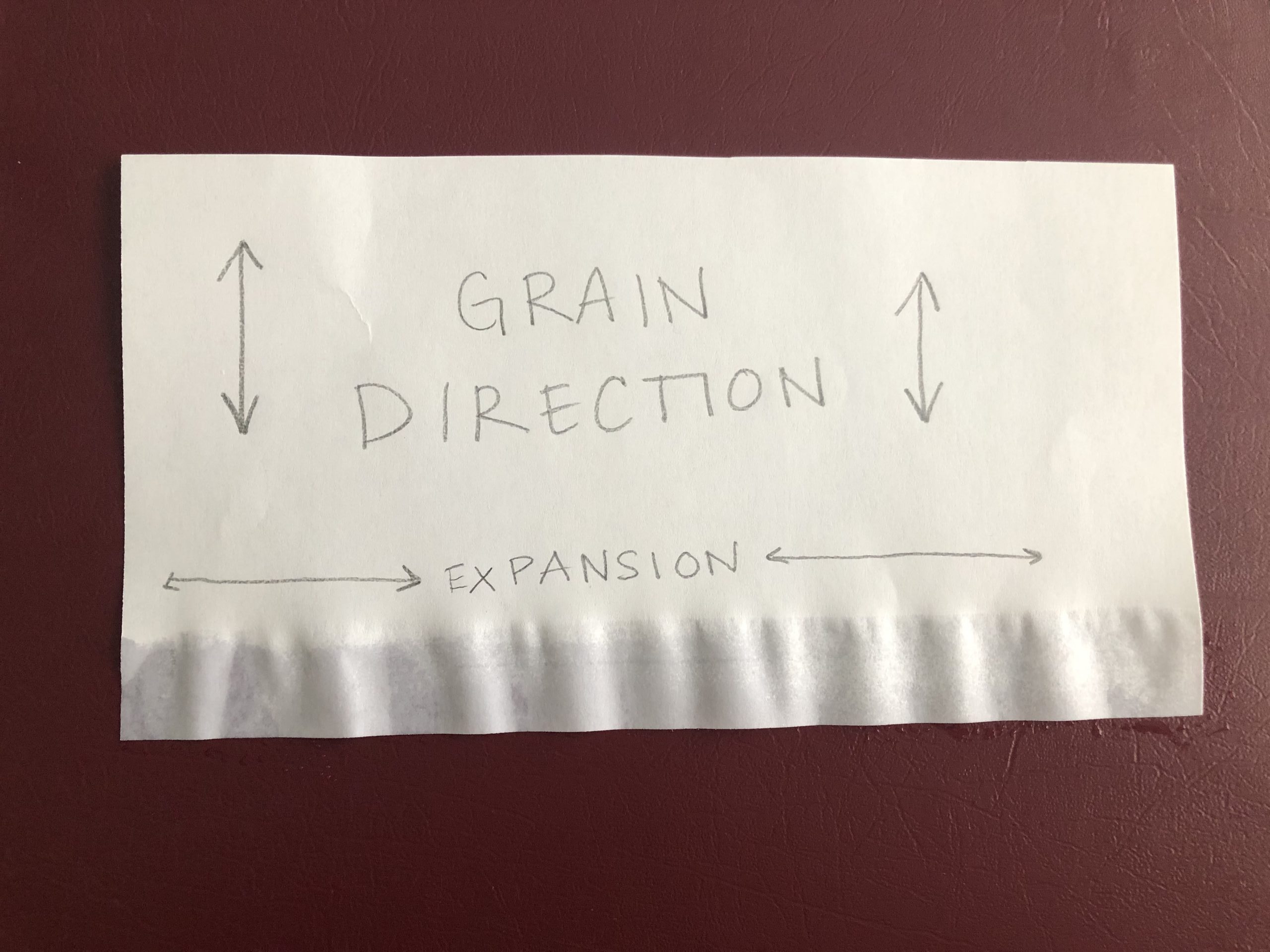
That means if the grain direction is vertical on a given sheet of paper – that is, many paper fibers are oriented vertically within the sheet – when the sheet gets wet it will actually get wider across the horizontal dimension. Depending on the papermaking process and technique, some papers will expand more than others, and the expansion can actually be quite dramatic.
The water used for papermaking can also be responsible for the chemical makeup of the paper by way of additives or impurities. The quality of the water is important since the chemicals present will usually end up trapped inside the finished sheet of paper. Poor quality water can leave metallic particles – most commonly iron or copper – which cause damage to the paper as they age and corrode. Other water sources may contain calcium, which will act as a neutralizing agent for acids formed by the paper during aging, thereby internally stabilizing the sheet over time.
In short, water is a central and indispensable part of paper from the get go, and has a major impact on its inherent properties.
Humidification – Loosen up!
One of the most common ways that conservators make use of water for the treatment of paper is by way of humidification. Humidification introduces water into the paper in vapor form (sometimes mist or fine droplets are also used for a faster result) to increase the overall water content. The goal of humidifying a sheet of paper is to take advantage of the changes (i.e. breaking or loosening) of bonds both within and between fibers within the paper. With more water present in the paper, the overall structure becomes more flexible and relaxed, and the fibers can more easily move past one another. Macroscopic features like wrinkles, folds, or curling, which were “locked” into the paper due to the way the fibers were bonded to each other, can now be manipulated out with careful handling.
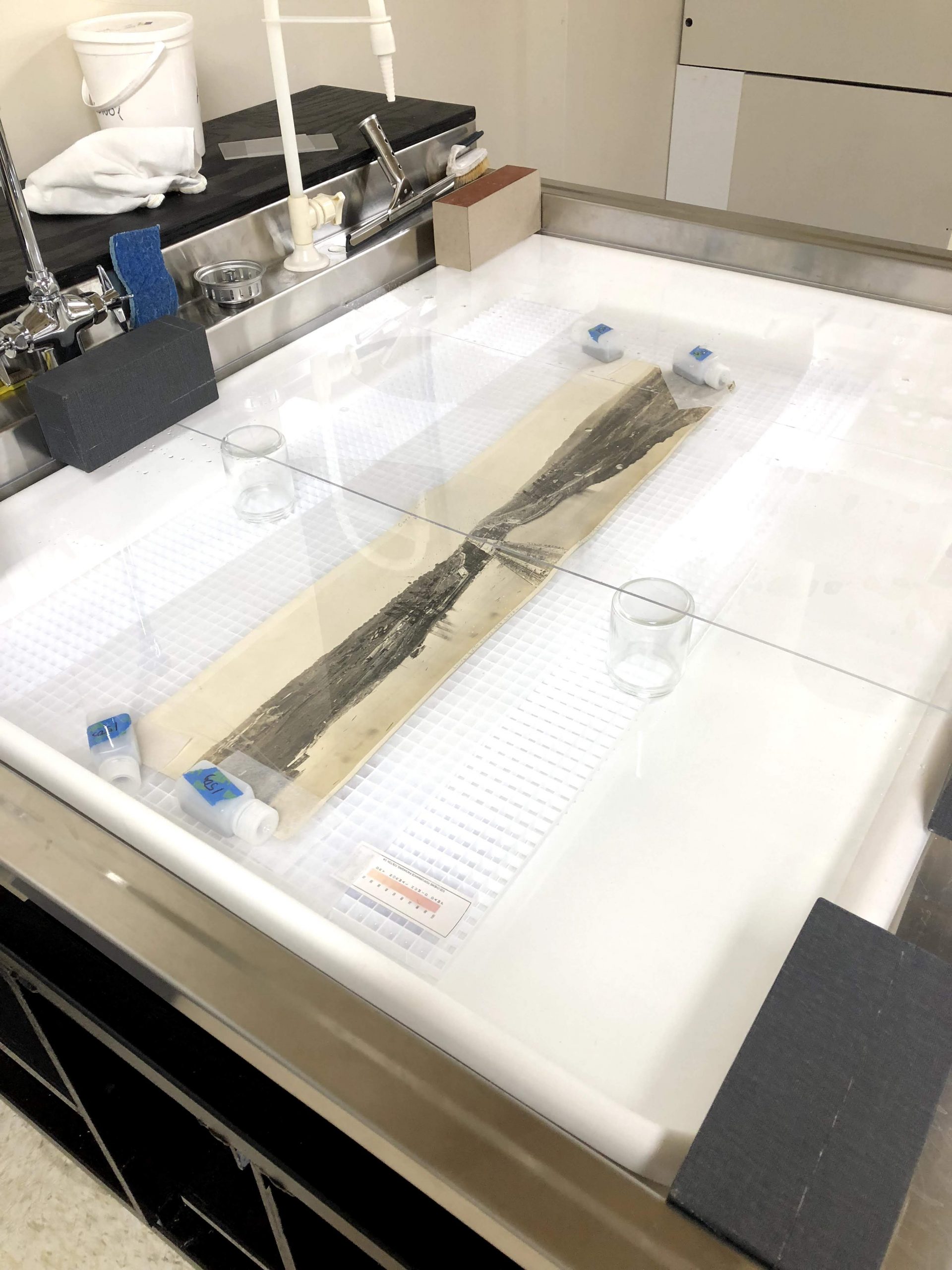
Controlling how the water exits the paper while drying is just as important as the initial wetting in these types of treatments. Ensuring the sheet is in the correct position or shape while drying will encourage bonds to form and strengthen in the right places so that shape is maintained when dry. Since the desired result is almost always to flatten unwanted distortions, drying is normally done under some weight to prevent too much free movement of the sheet as it contracts during drying. The images below show the use of humidification to flatten a rolled panoramic photograph from the TMMP collection (“Construction of the Panama Canal,” 1912, silver gelatin).


Dangers of Uncontrolled Humidification
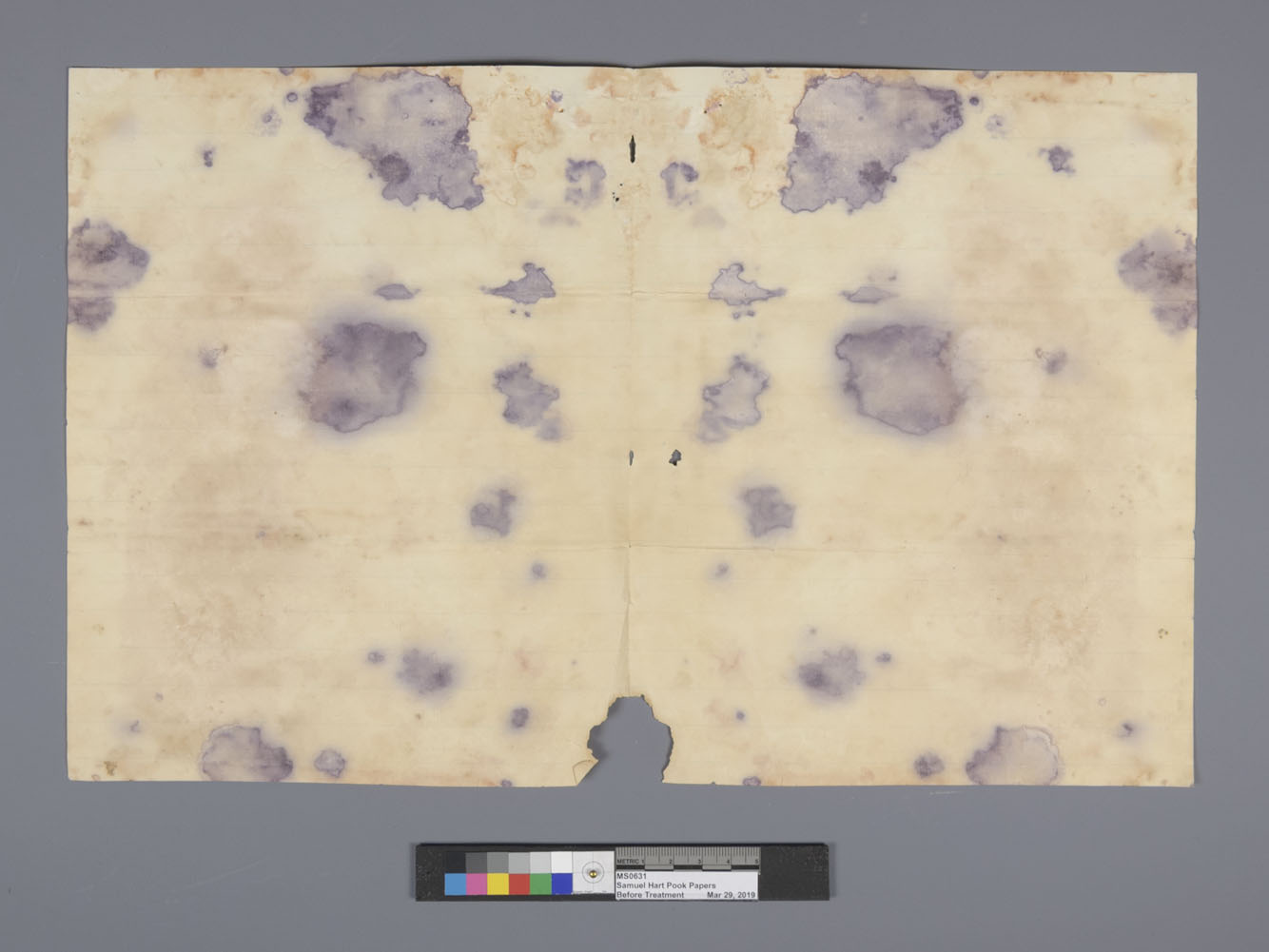
The entire process of humidification and subsequent drying is carefully managed during treatment, since uncontrolled humidification can lead to a number of problems. The first is the same process described above for flattening paper, but with the opposite effect. Paper rarely will “choose” to be flat when allowed some freedom, especially if it cannot wet or dry evenly because it is partially constrained in some way, such as the pages in a book or a print taped to a backing board around the edges. Excess humidity can also damage paper by encouraging natural aging reactions to occur. Water is a necessary ingredient in the chemical reactions that break down the cellulose in paper, and when in a humid environment, these reactions occur faster, causing the paper to become brittle and yellowed. Finally, an extended humid environment creates an ideal breeding ground for mold, which will eat away at the paper and sometimes cause significant and irreversible staining.


Bathing – Better Out Than In
Bathing is another common practice in paper conservation involving water. The main difference between humidifying and bathing is the amount of water used and the format in which it is delivered into the paper. For humidification, water is delivered in vapor form, slowly, until the paper is just relaxed. For bathing, water is introduced in liquid form, and fully saturates the sheet.
The purpose of bathing is to move materials into and out of the paper. As paper ages, the cellulose and other components break down into acidic chemical byproducts. These byproducts are yellow to brown in color, which is why old paper can start to look darker over time. Furthermore, the presence of the acidic components in the paper encourages further chemical degradation to occur, making the paper break down even faster. Fortunately for paper conservators, these pesky byproducts are soluble in water.
The function of bathing is dependent on the concept of diffusion. Diffusion is the net movement of solubilized materials in solution from an area of high concentration to an area of low concentration to achieve equilibrium, or even distribution. When a piece of paper is submerged in plain water, there is a much higher concentration of acid components in the paper than in the water, so the acids are drawn out into the bath water until there is an equilibrium. The bath water can then be replaced with clean water multiple times, drawing out more and more acids, until there is little to none left in the paper. The result is a cleaner, brighter, and more stable piece of paper.
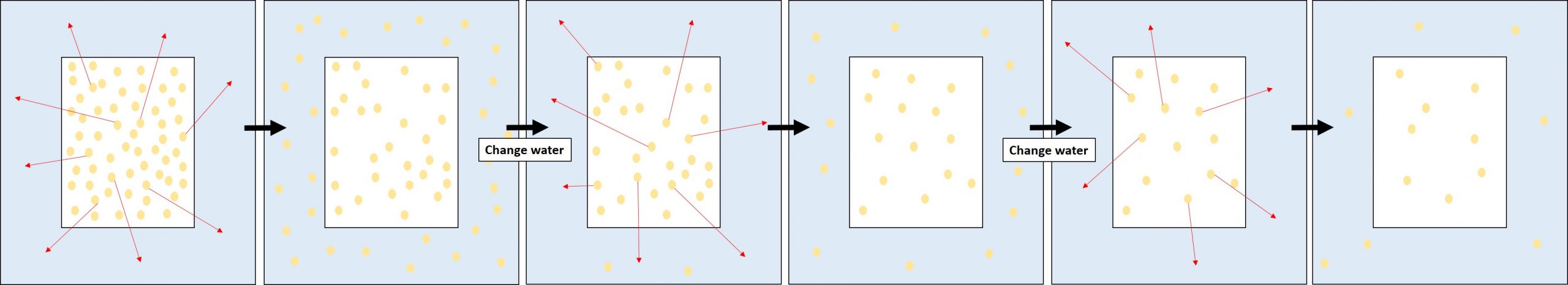

The idea can be used in reverse, as well. In some cases, it is desirable to put an alkaline material, such as calcium hydroxide, into the paper to neutralize acids as they form over time. To accomplish this, calcium hydroxide is dissolved in the bath water. With the concentration of calcium hydroxide in the bath being higher than that in the paper, the calcium hydroxide will move into the paper until there is an equilibrium.
In addition to moving things in and out of the paper, the presence of water helps reestablish some chemical bonds between paper fibers that may have broken over time due to acidic breakdown, which leaves the paper less brittle and more flexible after bathing.
Dangers of Uncontrolled Water Exposure
Now, your instinct may still be telling you that throwing an important document or print into a tub of water is a recipe for disaster, and in many ways, your instinct is absolutely right! Uncontrolled exposure to liquid water can cause a whole host of issues for a piece of paper. One of the most obvious would be bleeding inks or other media. A great deal of testing happens before a conservator even thinks about bathing paper, not only to protect visible components like media, but also less obvious ones like sizing or brightening agents. In the end, some objects simply cannot be bathed safely. However, in some cases it is possible to change the solubilizing effect of water by adding solvents or changing the pH (making it more alkaline or acidic).
In other cases, the method of applying the water can be changed to avoid harming the media. One of many such bathing techniques used to control the effects of water is suction. In this method, the water is applied over a vacuum which immediately draws the water through the paper, decreasing the amount of time the liquid water is suspended in the paper, giving it less time to do damage.


Uneven wetting and drying of a piece of paper can result in a wrinkled surface called “cockling” as the paper expands and contracts. Furthermore, it can result in stains called “tidelines,” which most people would refer to generically as “water stains.”

When only a small area of paper gets wetted, the acidic byproducts present in the paper are solubilized and can move freely within that area. When the water evaporates, it does so starting from the outer edges of the wet area and moving inward. Since the water is evaporating, but the acids are not, they are left in a high concentration at the edge of the wet spot, thus leaving a stain. To avoid tidelines, conservators try to use water overall rather than locally when possible, or carefully control the drying and evaporation of the water if working in a small area.

Finally, a paper that is saturated with water is naturally weakened and can easily tear during handling. (Think about trying to pick up a wet tissue!) To make it possible to move the paper when wet, conservators use a support sheet (usually a polyester webbing material) to avoid handling the paper directly and causing physical damage.
Water is an important and powerful substance which, left unchecked, can cause all sorts of problems for a delicate material like paper. However, with an understanding of the underlying interactions between paper and water, its power can be harnessed and used for good! Obviously the use of water in paper conservation goes way beyond humidification and bathing, but these techniques make use of water in a raw and direct fashion, which tends to surprise those unfamiliar with the field.
Now, I may not be using quite as much water as the Monitor conservators, but maybe we’re not so different? After all, it’s water that connects us!