The Plastics Age
History is filled with ages that are tied to the innovation of materials: The Stone Age, The Bronze Age, and The Iron Age. We are currently in The Plastics Age. Plastics have changed so much in our daily lives. Plastics are around us all the time. They are in every electrical thing in our houses, in the clothes that we wear, in our furniture and the packaging of our food.
This means that as caretakers of historic objects, museums have to consider how long plastic materials will last in our collections. We focus on what we have to do and learn in order to care for plastic objects. We also study plastics in order to store them in ways that better ensure their survival. This is a complicated thing. Plastics are not simple materials, and what works for one may damage another. Some plastics have been around longer than others, so we know more about them. We can see how they’ve aged. For other plastics, we can guess at how they will survive (or not) based on their behaviors and chemistries, while still others are a gigantic question mark.
There are so many plastics, and so many questions that I’d like to cover. Eventually. Given enough time. But for today I’d like to time-travel a bit. I’d like to go back to the 1800’s, when science and trade were first beginning to look at a material that we are all familiar with today. A naturally occurring polymer that pioneered, in so many ways, our relationship with plastics.
Chapter 1: Rubber
Note: All written accounts of the use and evolution of rubber I have read come from European and North American (US) authors. It is vital to note that there is a long history of the use of tree sap (latex) for products (shoes and sporting goods to name two) in South and Central America as well as in Asia where the trees are found that produce the base polymers for rubber, 1,4-cis-isoprene or 1,4-trans-isoprene. The use of the raw materials by European and North American markets started because of trade with cultures in South and Central America as well as Asia. There was no such thing as waterproof boots or coats in Europe or the USA until someone saw and traded for rubber shoes made by people in Brazil.
Rubber is a plastic that was initially made using the dried latex taken from one of many different species of tree. There are numerous names for this latex product including “gutta percha”, “india-rubber” and “caoutchouc” (pronounced cowt-choo, or at least that’s how I pronounce it).

© User:Iamshibukc / Wikimedia Commons / CC-BY-SA-3.0
Chemically, rubber is made from one of two polymeric backbones, either 1,4-cis-isoprene or 1,4-trans-isoprene. Which polymer backbone is found depends on the original source plant. The two materials produce physically indiscernible products.
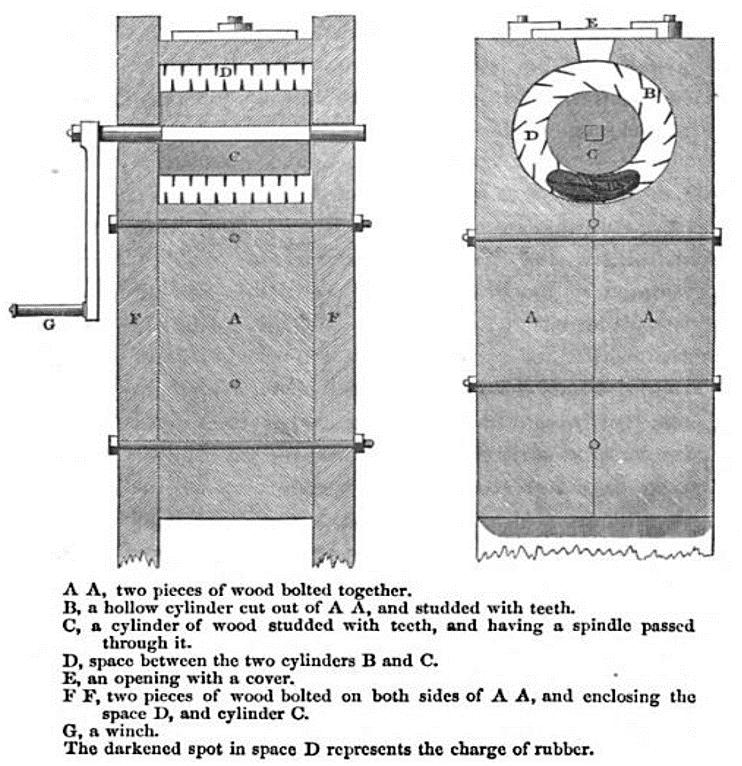
Natural rubber was processed in the 1800’s using a method called mastication. (A British patent for this process dates to 1820.) Rubber mastication mechanically breaks it up, consequently heating it a bit, until the rubber eventually becomes a uniform material.
The color of natural rubber is the color of a rubber band, which is one product that is still often made from natural rubber because of its superior elasticity.
Carbon black was and is commonly added to reduce the effects of light on the material (the coloring protects it from breaking down). Early in rubber production other dyes were added, allowing a beautiful array of colorful products.
Natural rubber is sensitive to changes in humidity and temperature. It becomes tacky and chemically breaks down in hot humid climates. It also becomes brittle in the cold. Environmental climate control for natural rubber is a key element of care.
Natural rubber was used to create many objects in Britain, which being an island nation has a more temperate climate. Attempts were made to sell the first waterproof natural rubber footwear in the United States in the 1830s. However, a hot and humid summer quickly soured consumers as they found that the shoes became gummy, sticky and eventually smelly.
Charles Goodyear in the United States became obsessed with finding a way to make rubber usable at every temperature and humidity. There are some excellent accounts of this obsession and I’ve linked to one here. I also was very interested to learn that the Goodyear Company is not tied to Charles Goodyear, but that is another story that you can learn about on their page.
Charles Goodyear found that the addition of sulfur to rubber created a material that didn’t break down in heat and humidity and didn’t become brittle in the cold. It lost its elasticity, but retained some plasticity depending on how much sulfur was added. He patented his “vulcanization” processes in the United States (1844, 1851), however only his descendants saw the proceeds of this.

Vulcanized rubber is more robust a material than natural rubber. However, the presence of sulfur in the rubber can cause problems as it ages, oxidizes and generally breaks down. Formation of chemicals like hydrogen sulfide and sulfuric acid are possible from vulcanized rubber, and these chemicals are dangerous to nearby objects, with sulfuric acid able to damage the rubber object itself.
Note: Don’t store your silver near vulcanized rubber, it will tarnish much more quickly.
Both natural rubber and vulcanized rubber materials oxidize over time in normal environmental conditions. This oxidation causes the material to become brittle, may cause cracking, and the surface becoming “dusty” or sticky (depending on the object and its environment). The best storage for rubber is in a cool anoxic environment with no or low light.
Unintentional but Good Rubber Storage
One place where there is low light, low oxygen, and it is pretty cool (both physically and metaphorically) is a wreck-site at the bottom of the ocean.

The USS Monitor wreck preserved many rubber objects.



As a research scientist, I am working with our team of conservators to document the chemical fingerprints of our rubber objects using our attenuated total-reflectance-Fourier transform infrared spectrophotometer (ATR-FTIR).
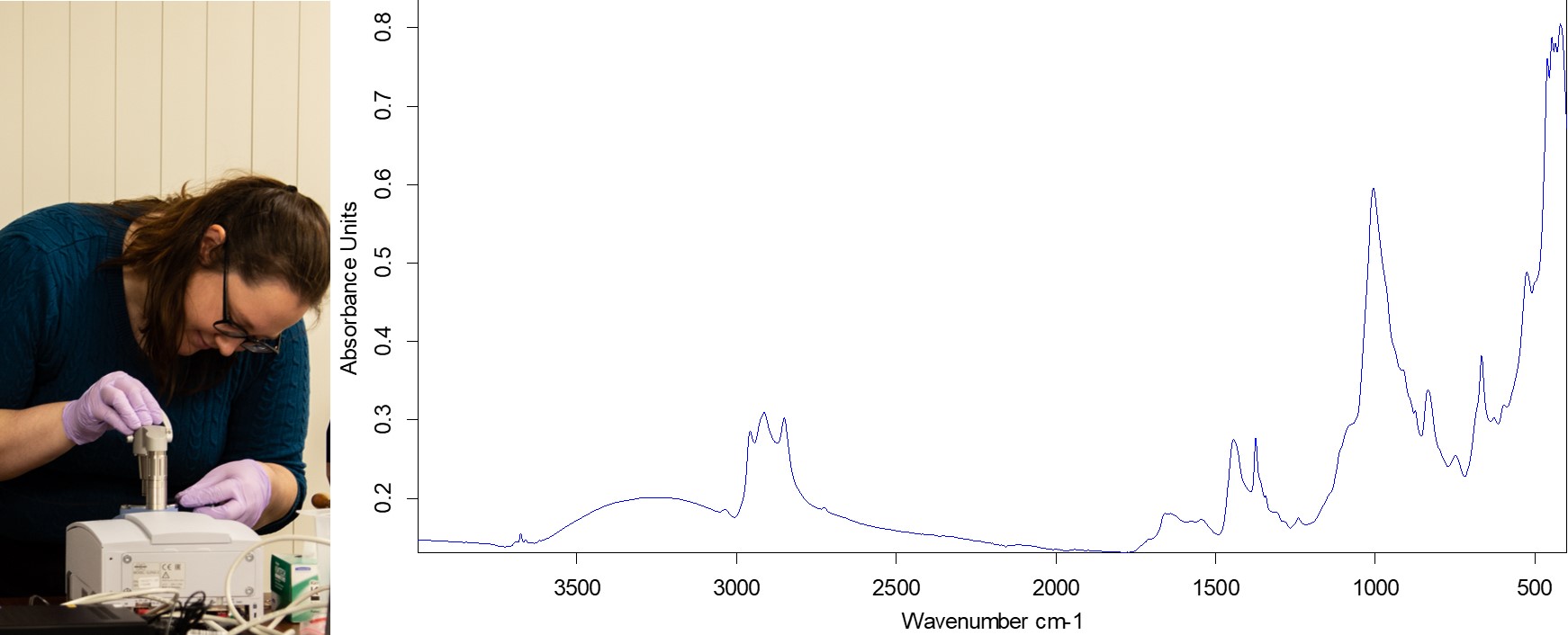
ATR-FTIR allows me to document the type of rubber that we are seeing, whether it is 1,4-cis or 1,4-trans isoprene, as that identifies which plants were potential sources and may help us understand trade routes during the Civil War. I am also looking to track how much oxidation we are seeing in these objects by monitoring the formation of epoxide and peroxide species.
The spectrum of the gasket above allows me to identify the rubber as 1,4-cis-isoprene, typing it to a South or Central American species of tree. I also found a tell-tale peak for epoxide, meaning that the rubber is undergoing oxidation. However the spectrum overall looks pretty good for a 150-160 year old rubber.
This is on-going research and it has already shown me how well some of our rubber objects have survived the last century and a half. My hope is that this work will also allow future researchers a window into each of these objects’ chemical status at this moment in time.
This is why I love scientific research of museum objects. I get to connect with the past, tell y’all now about the cool things we discover, and pass the informational baton off to the people who come next.